Solved isothermal layer of the clapeyron gas remains in rest Diagramme de clapeyron (a) ideal cycle clapeyron diagram [14] and (b) real heat storage system
(a) Schematic and (b) Clapeyron diagram for an ideal simple adsorption
Processos isotèrmics i què signifiquen
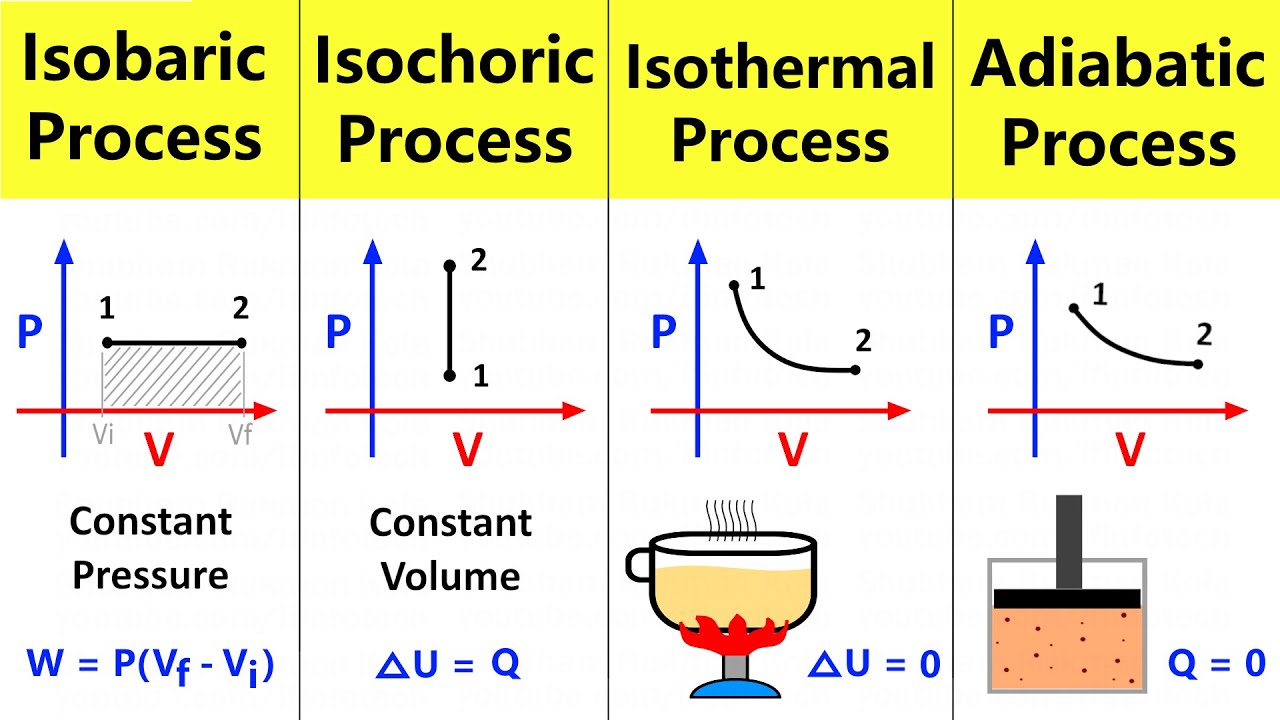
Thermodynamic processes: isobaric, isochoric, isothermal and adiabatic
Clapeyron diagram for the theoretical ctar thermodynamic cycle [33Clausius clapeyron equation Clapeyron binaryClapeyron diagram for the basic adsorption ice production thermodynamic.
(a) ideal cycle clapeyron diagram [14] and (b) real heat storage systemClapeyron physisorption refrigeration Appropriate correct clapeyron equationClapeyron equation.

Isothermal process
Explain in detail the isothermal process.Isothermal process Isothermal thermodynamic processes thermodynamics tecIsothermal process in a closed system.
Clapeyron equation clausius process ppt powerpoint presentation adiabatic slideserveIsochoric process photos and images & pictures Web diagram clapeyron muddiest points chapter figure unified mit notes eduPlace the correct labels on the appropriate area of the phase diagram.

Phase diagrams. iv. the clapeyron equation
Clapeyron cycle sketch diagram of binary mixture (x e ).(a) schematic and (b) clapeyron diagram for an ideal simple adsorption Formule de clapeyronIsothermal process.
Isothermal process (constant temperature process)8.9 muddiest points on chapter 8 Clapeyron diagram for two cold generation sources in the solid sorptionReal clapeyron diagram..
Clapeyron equation
Adsorption clapeyronClapeyron sorption cooler cold Clapeyron clausius equation phase seu derivation example diagramsDiagramme de clapeyron.
Clapeyron diagram of the solid sorption cooler with heat recoveryClapeyron diagram of an ideal adsorption cycle Clapeyron diagram of heat pump with two sources of cold.Phase diagrams.

Clapeyron diagram for a single stage physisorption refrigeration cycle
Clapeyron diagram for the theoretical ctar thermodynamic cycleDiagrama de clapeyron Isothermal process temperature constant relationship internal energy engineering change.
.






